Source: MHRA /Gov.uk 23.4.24
(Cheplapharm – Canadian Livery), EL(24)A/14: Neon Healthcare Ltd is recalling the specific batch mentioned in this notification as a precautionary measure.
MDR number
MDR 191-04/24
Company name
Neon Healthcare Ltd
Product name
Suprefact 1 mg/ml solution for injection (Cheplapharm – Canadian Livery)
PL N/A
SNOMED Code
N/A
| Batch Number | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| 3F022A | 12/2024 | 2 multi-dose vials of 5.5ml | 11 April 2024 |
Active Pharmaceutical Ingredient: Buserelin Acetate
Brief description of the problem
Neon Healthcare Ltd is recalling the specific batch mentioned in this notification as a precautionary measure. This is because the named batch of Suprefact 1mg/ml solution for injection is being distributed in packaging intended for the Canadian market by Cheplapharm, instead of the correct UK packaging by Neon Healthcare Ltd for Buserelin 1 mg/ml solution for injection.
Advice for healthcare professionals
Stop supplying the above batch immediately. Quarantine all remaining stock and return it to your supplier using your supplier’s approved process.
Wholesalers are also requested to check stock of Buserelin 1 mg/ml solution for injection for any packs that match Suprefact 1 mg/ml solution for injection (MAH: Cheplapharm – Canadian Livery) as per the product images below.
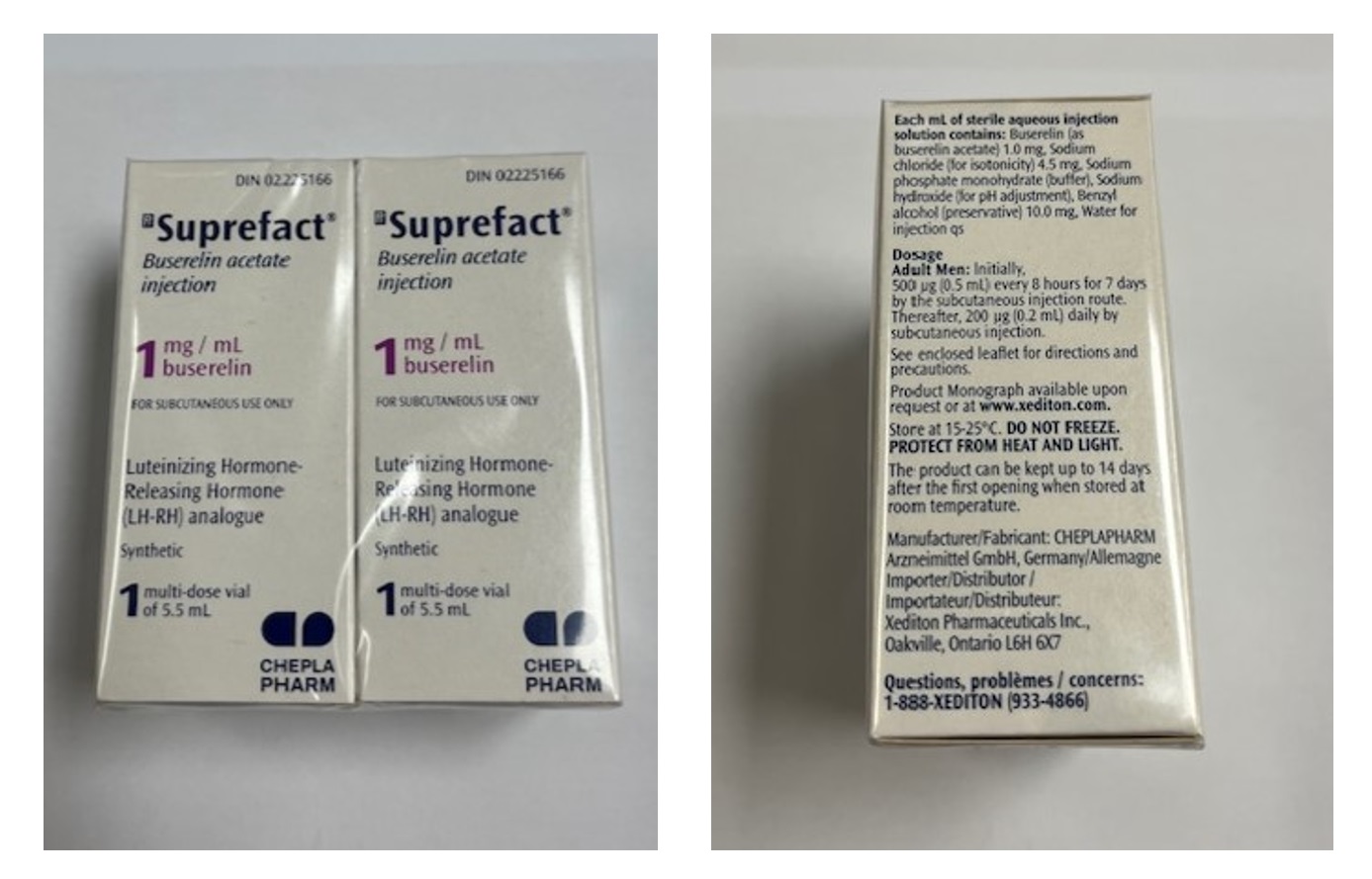
Advice for patients
No further action is required by patients as this is a Pharmacy and Wholesaler level recall. Patients who experience adverse reactions or have any questions about the medication, should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Further information
For medical information or stock control enquiries please contact: Neon Healthcare Ltd via Medinfo@neonhealthcare.com or +44 (0) 1992 926 330
Recipients of this Medicines Recall should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists and dispensing general practitioners for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Download document
Click here for the source.

